Our Research in Neurocardiology
We work at the interface of Cardiovascular Science and Neuroscience. We utilize cutting edge tools in both of these areas to decipher patterns and mechanisms underlying health and diseased Cardiac-Neural interactions. Our studies involve animal models and humans to maximize the potential to translate our work to the bedside for therapeutic benefit for patients.
The cardiac neuraxis (nervous system) undergoes remodeling, and becomes dysfunctional following myocardial infarction (MI), chronic cardiomyopathy (CMY), and other structural heart diseases.
Altered neuraxial function can initiate and/or maintain ventricular arrhythmias via various mechanisms. Interventions targeting the cardiac neuraxis hold great potential and have been used clinically to treat VAs, particularly in patients with hereditary heart rhythm disorders and structurally abnormal hearts. Clinical applications of cardiac neuraxial modulation at the level of spinal cord, stellate ganglion, and peripheral sympathetic and vagus nerve are increasing in use and development
Examples of thematic areas in our lab are:
Neuromodulation for Cardiac Arrhythmias and Heart Failure
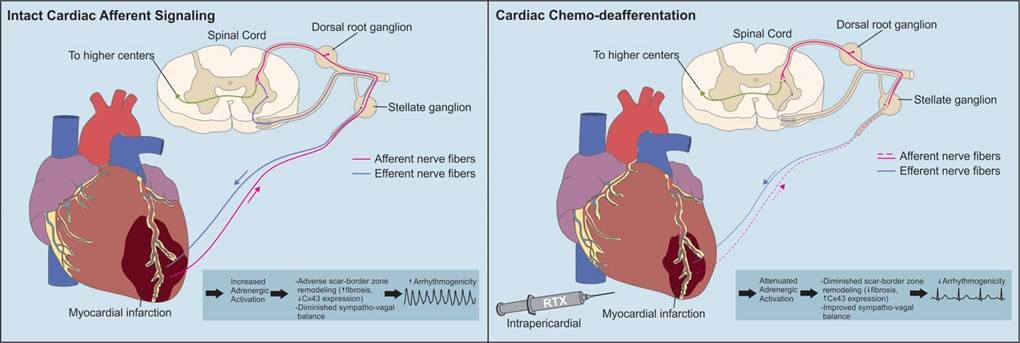
The peripheral nervous system is central to the pathophysiologic sympathoexcitation that worsens the heart after initial injury. This progressive worsening of cardiac function is partially responsible for creating autonomic imbalance. The resulting arrhythmias are common and are thought be responsible for the vast majority of sudden cardiac deaths worldwide (almost 12 million deaths/year worldwide). Our lab is interested in understanding how to harness the regenerative power of the nervous system to repair the heart after injury. We use a variety of strategies such as chemo-axotomy (see below), vagal nerve stimulation, and other approaches as neuromodulation strategies to treat the heart.
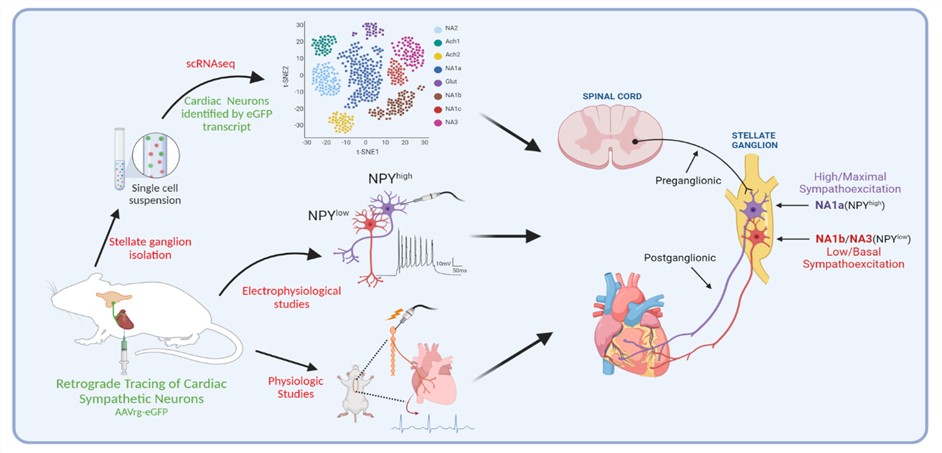
Deciphering Cardiac Sympathetic Control at Single Cell Resolution
The stellate ganglion, an amalgamation of the C8 and T1 ganglion within the sympathetic chain, as well as the middle cervical ganglia house post-ganglionic sympathetic neurons directly innervating and controlling the heart. Our group is interested in understanding the subtypes, roles, and functions of individual neurons within the stellate ganglion at single cell resolution. We investigate the roles of these cells in regulating the heart using optogenetic, chemogenetic, and intersectional genetic approaches of cardiac neural circuits mediated by these neurons.
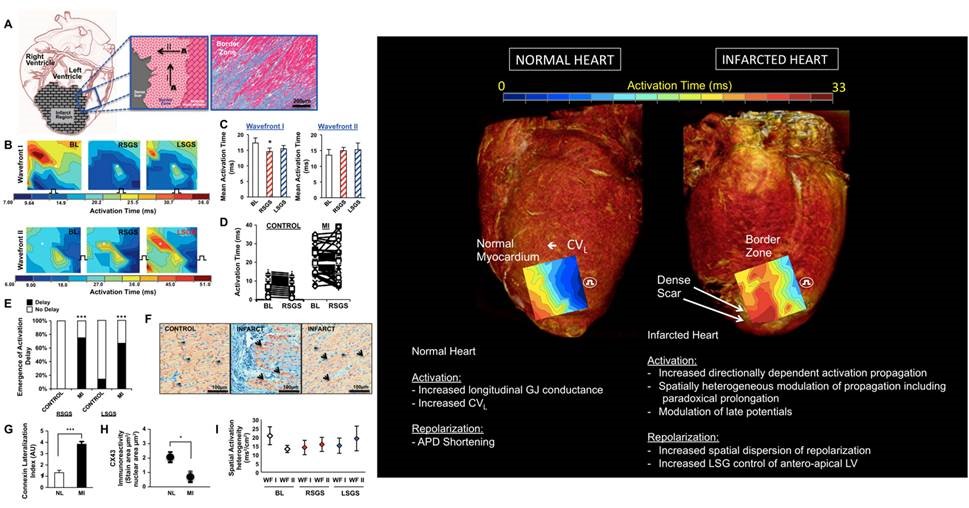
Contributions of Altered Scar-Border Zone Sympathetic Nerves to Lethal Ventricular Arrhythmias
Intramyocardial nerves exert powerful control on myocardial excitability both in physiologic and pathophysiologic states. In the context of myocardial infarction, the distribution of nerves in the scar border zone (region between the dense scar and surviving cardiomyocytes at the edge of the infarct) becomes heterogeneous and amplifies the spatial dispersion of electrical properties (activation and repolarization). During enhanced sympathetic states, monomorphic ventricular tachycardia circuits become altered in a manner that facilitates the initiation and maintenance of ventricular arrhythmias. We are interested in deciphering the mechanisms by which these nerves become altered and perturb electrical function leading to lethal ventricular arrhythmias. These lethal arrhythmias are responsible for 12 million deaths around the world annually.
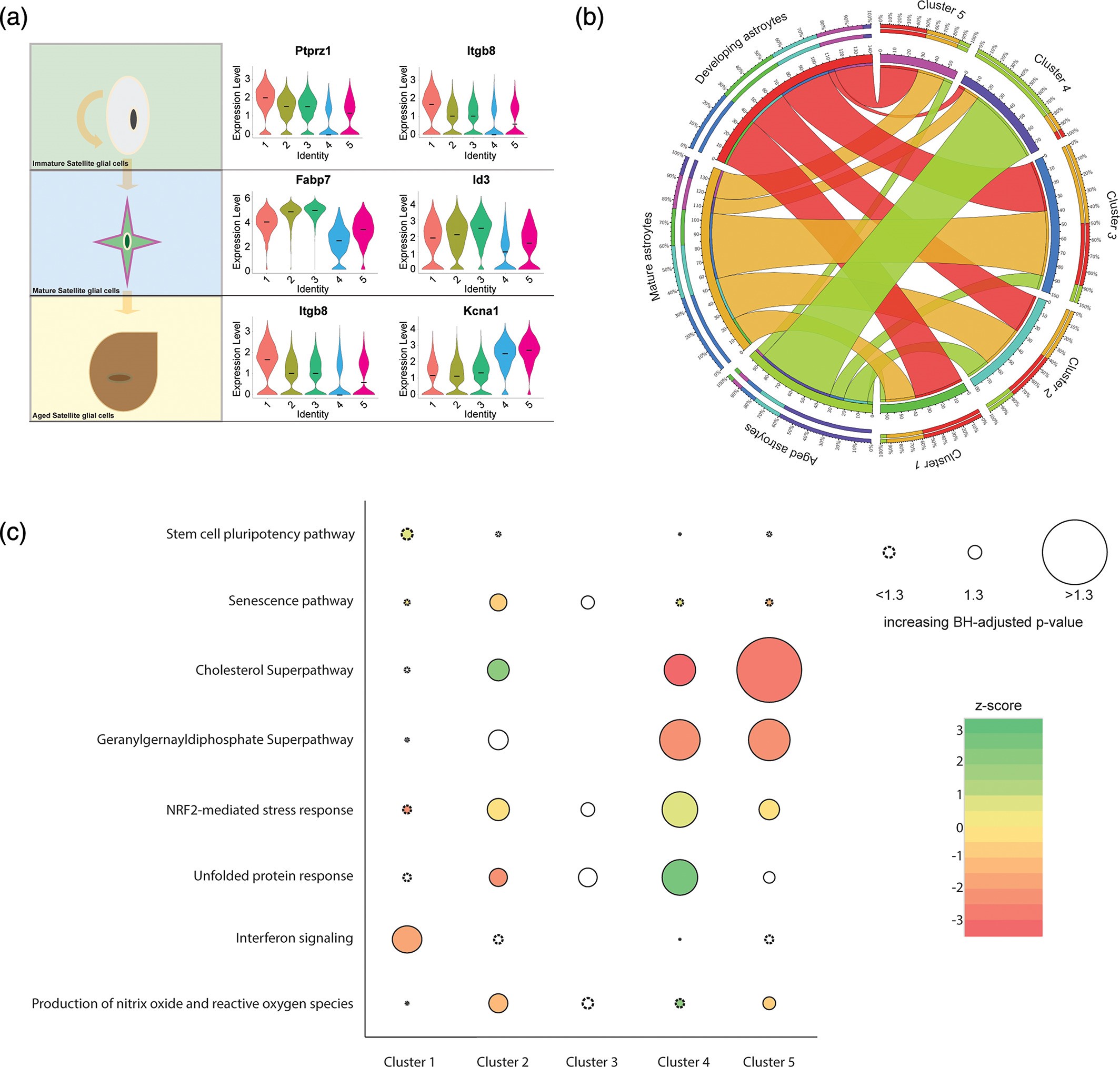
Satellite Glial Activation as a Driver of Sympathetic Neuronal Dysfunction and Maladaptive Cardiac Sympathoexcitation
Satellite glial cells, analogous to astrocytes in the peripheral nervous system, are increasingly recognized to serve important roles in physiologic and pathophysiologic functions of neurons. Our lab is currently funded by NIH R01HL167885 to investigate satellite glial cell activation as a pathogenic factor in sympathetic dysfunction responsible for ventricular arrhythmias and progressive cardiomyopathy and heart failure.
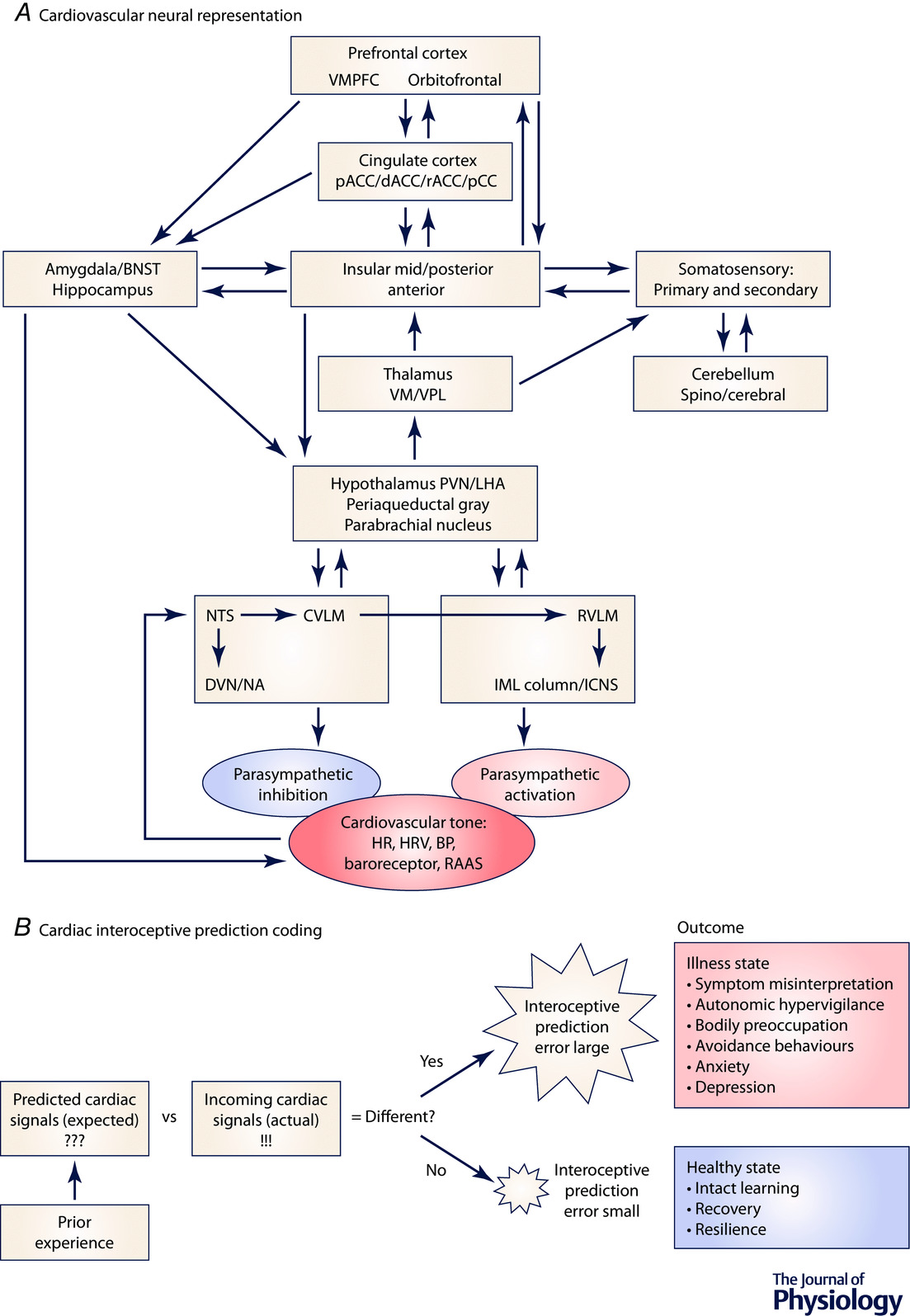
Influence of Myocardial Injury on Cardiac Interoception & Behaviour
The field of interoception is rapidly gaining steam. Interoception refers to the process by which the brain sense the state of its internal organs, and how that influences brain function, behavior, and efferent responses. There are strong links between interoceptive awareness and decision making, as well as conditions such as anxiety and stress. The relationship between cardiac interoceptive awareness and decision making, anxiety, stress etc are just beginning to be understood. We are looking to expand our work in this area using murine models and importantly in humans. Join us!